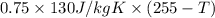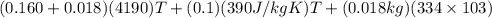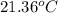A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal equilibrium at atmospheric pressure.
Part A
If 0.750kg of lead at a temperature of 255 c is dropped into the calorimeter can, what is the final temperature? Assume that no heat is lost to the surroundings

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 19:00, MIYAISSAVGE2409
Like a key in a lock, the shape of the. must bind with the. of the recieving neuron
Answers: 1

Physics, 21.06.2019 21:30, krystalhurst97
Astudent looks at ocean waves coming into the beach. an ocean wave with more energy will a) have a greater height. b) have a greater period. c) travel toward the beach faster. d) strike the beach with greater frequency.
Answers: 1


Physics, 22.06.2019 18:20, shongmadi77
Wavelength of 125 meters is moving at a speed of 10 m/s. what is it's frequency?
Answers: 1
Do you know the correct answer?
A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal e...
Questions in other subjects:

Chemistry, 05.01.2021 01:50


Chemistry, 05.01.2021 01:50


SAT, 05.01.2021 01:50

Mathematics, 05.01.2021 01:50

Mathematics, 05.01.2021 01:50



Arts, 05.01.2021 01:50


 ) = 0.1 kg
) = 0.1 kg
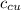 ) = 390 J/kg K
) = 390 J/kg K
 ) = 0.160 kg
) = 0.160 kg
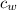 ) = 4190 J/kg K
) = 4190 J/kg K
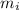 ) = 0.018 kg
) = 0.018 kg

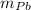 ) = 0.75 kg
) = 0.75 kg
 ) = 130 J/kg K
) = 130 J/kg K