Physics, 12.02.2020 06:02, djfluffyman999
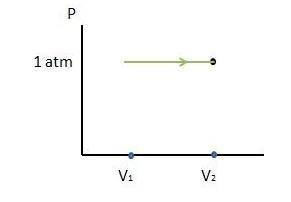
A cylinder contains 0.250 mol of carbon dioxide (CO2) gas at a temperature of 27.0∘C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0∘C. Assume that the CO2 may be treated as an ideal gas. (a) Draw a pV-diagram for this process. (b) How much work is done by the gas in this process? (c) On what is this work done? (d) What is the change in internal energy of the gas? (e) How much heat was supplied to the gas? (f) How much work would have been done if the pressure had been 0.50 atm?

Answers: 3
Other questions on the subject: Physics


Physics, 22.06.2019 23:30, olivasm5626
Macy always thought there were only a few hair colors blonde brown and black however when she actually began looking around she saw varying shades of these hair color what is a possible reason for so many different hair colors
Answers: 1

Physics, 23.06.2019 01:00, pulliamdylan
What electron configurations do atoms usually achieve by sharing electrons to form covalent bonds?
Answers: 3
Do you know the correct answer?
A cylinder contains 0.250 mol of carbon dioxide (CO2) gas at a temperature of 27.0∘C. The cylinder i...
Questions in other subjects:

English, 29.03.2021 05:40


Mathematics, 29.03.2021 05:40



English, 29.03.2021 05:40

Mathematics, 29.03.2021 05:40


Social Studies, 29.03.2021 05:40


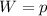 ΔV ------------------------ (i)
ΔV ------------------------ (i)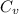 ΔT -------------------- (iv)
ΔT -------------------- (iv)