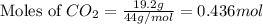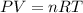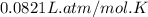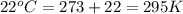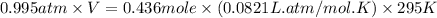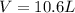Physics, 11.02.2020 23:31, kiarabermudez754
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus. Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constant temperature of 22 degrees C. Assume that the initial volume of dry ice is negligible and that CO2 behaves like an ideal gas.

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 04:20, pippalotta
Astone is thrown into a pond. what happens to the amplitude of the resulting waves as they get farther from the point where the stone hit the water? explain.
Answers: 3

Physics, 22.06.2019 10:00, kaniyawilhite
If a stone with an original velocity of 0 is falling from a ledgeand takes 8 seconds to hoybthe ground whays the final velocity of the stone
Answers: 2


Physics, 22.06.2019 14:40, viktoria1198zz
14. a body is projected with velocity vi from a. at the same time another body is projectedvertically upwards from point b withvelocity v2 lies vertically below the highestpoint. both the bodies collide thenv2÷v1is
Answers: 1
Do you know the correct answer?
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus...
Questions in other subjects:

English, 10.12.2021 23:10

English, 10.12.2021 23:10





Mathematics, 10.12.2021 23:10




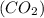 .
.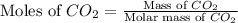
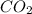 = 44 g/mole
= 44 g/mole