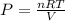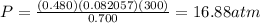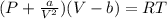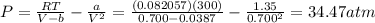Assume that you have 0.480 mol of N2 in a volume of 0.700 L at 300 K .
1. Calculate the...

Physics, 11.02.2020 05:28, eweqwoewoji
Assume that you have 0.480 mol of N2 in a volume of 0.700 L at 300 K .
1. Calculate the pressure in atmospheres using the ideal gas law.
2. Calculate the pressure in atmospheres using the van der Waals equation. For N2 , a=1.35 (L2⋅atm)/mol2 , and b=0.0387 L/mol

Answers: 2
Other questions on the subject: Physics


Physics, 23.06.2019 18:00, SmartScholar4094
Diego kicks a soccer ball from the end line. his fellow students time and mark the soccer ball as it moves down the field. the graph represents the ball's progress.
Answers: 3

Physics, 23.06.2019 22:50, jared2461
The steel pipe ab has a 102-mm outer diameter and a 6-mm wall thickness. knowing that arm cd is rigidly attached to the pipe, determine the principal stresses and the maximum shearing stress magnitude at point k. (round the final answers to one decimal place.) given: f = 10 kn
Answers: 3
Do you know the correct answer?
Questions in other subjects:



Mathematics, 08.03.2021 20:40



Mathematics, 08.03.2021 20:40


Mathematics, 08.03.2021 20:40



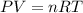 (1)
(1)