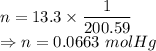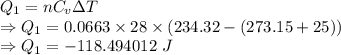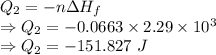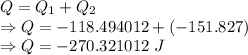Physics, 21.01.2020 03:31, haftjnd9156
Calculate the heat energy released when 13.3 g of liquid mercury at 25.00 c is converted to solid mercury at its melting point. constants for mercury at 1 atmheat capacity of hg(l) 28.0 j/(mol k)melting point 234.32 kenthalphy of fusion 2.29 kj/mol

Answers: 1
Other questions on the subject: Physics



Physics, 22.06.2019 11:00, dragon2565
Looking at this barometer, is the air pressure high or low? what type of weather would you expect? high, bad low, bad high, good low, good
Answers: 1

Physics, 22.06.2019 16:50, Jstylez1789
Consider the growth of a 20-nm-diameter silicon nanowire onto a silicon wafer. the temperature of the wafer surface is maintained at 2400 k. assume the thermal conductivity of the silicon nanowire is 20 wm-1k-1 and all its surfaces including the tip are subjected to convection heat transfer with the coefficient h = 1×105 wm-2k-1 and t∞ = 8000 k. when the nanowire grows to l = 300 nm, what is the temperature of the nanowire tip (t (x =
Answers: 1
Do you know the correct answer?
Calculate the heat energy released when 13.3 g of liquid mercury at 25.00 c is converted to solid me...
Questions in other subjects:




Mathematics, 22.06.2019 07:30




English, 22.06.2019 07:30



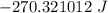
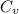 = Heat capacity of Hg = 28 J/mol
= Heat capacity of Hg = 28 J/mol = Change in temperature =
= Change in temperature = 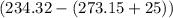
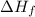 = Enthalpy of fusion = 2.29 kJ/mol
= Enthalpy of fusion = 2.29 kJ/mol