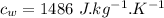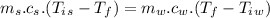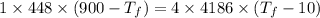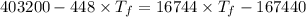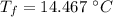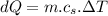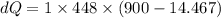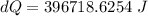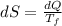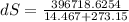Physics, 24.12.2019 06:31, haleyrene3924
A1.00-kg iron horseshoe is taken from a forge at 900∘c and dropped into 4.00 kg of water at 10.0∘c. assuming that no energy is lost by heat to the surroundings, determine the total entropy change of the horseshoe-plus-water system.

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 23:50, diana156
Select the correct answer from each drop-down menu. compared to its surroundings, the concentration of solutes is low inside a cell. so, the cell is with respect to its surroundings. a particular solute in this cell uses energy for its transport from the cell to its surroundings. this type of transport is called
Answers: 3

Physics, 22.06.2019 09:00, KitKat22Rose9
What is a possible result of higher air temperature caused by global warming
Answers: 1


Physics, 22.06.2019 12:30, mikurrjurdan
Matter is needed to transfer thermal energy bya. conductionb. convectionc. radiation d. both a & b.
Answers: 1
Do you know the correct answer?
A1.00-kg iron horseshoe is taken from a forge at 900∘c and dropped into 4.00 kg of water at 10.0∘c....
Questions in other subjects:









Mathematics, 10.08.2021 01:20


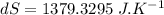
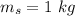 initial temperature of iron,
initial temperature of iron, 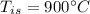 mass of water,
mass of water, 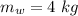 initial temperature of water,
initial temperature of water, 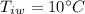
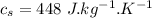 Specific heat of water,
Specific heat of water,