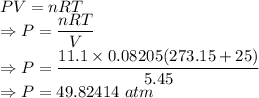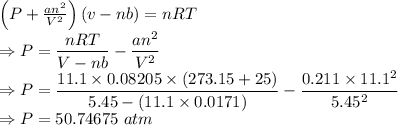Physics, 20.12.2019 01:31, moisealafleur
Calculate the pressure exerted by 11.1 moles of neon gas in a volume of 5.45 l at 25°c using (a) the ideal gas equation and (b) the van der waals equation. (for neon, a = 0.211 atm · l2/mol2 and b = 0.0171 l/mol.)

Answers: 2
Other questions on the subject: Physics

Physics, 21.06.2019 23:30, rainbowsauxe
Aquarterback pedals 3.3meters southward and then run 5.7meters northward. whats the distance ? and whats the displacement?
Answers: 2


Physics, 22.06.2019 05:30, justin5647
Which of the choices below is one of the primary gases found in the atmosphere? a. helium b. carbon dioxide c. nitrogen d. argon
Answers: 2

Physics, 22.06.2019 19:30, alexmarche8637
Contains chemical energy. a. heat b. light c. natural gas
Answers: 2
Do you know the correct answer?
Calculate the pressure exerted by 11.1 moles of neon gas in a volume of 5.45 l at 25°c using (a) the...
Questions in other subjects:

Chemistry, 24.09.2021 18:20

Physics, 24.09.2021 18:20

Chemistry, 24.09.2021 18:20


Computers and Technology, 24.09.2021 18:20


Mathematics, 24.09.2021 18:20


History, 24.09.2021 18:20