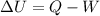Physics, 17.12.2019 02:31, prettydoll19
Acylinder, which is in a horizontal position, contains an unknown noble gas at 4.00 × 10 4 pa 4.00×104 pa and is sealed with a massless piston. the piston is slowly, isobarically moved inward 16.3 cm, 16.3 cm, which removes 1.50 × 10 4 j 1.50×104 j of heat from the gas. if the piston has a radius of 30.5 cm, 30.5 cm, calculate the change in the internal energy of the system δ u δu .

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 17:00, laurentsupreme
In a closed system, the total energy prior to an energy transformation is the total energy after. a. equal to b. unrelated to c. greater than d. less than
Answers: 2

Physics, 22.06.2019 03:50, am2garcia5
A30 kg weight lies on top of a massless piston of area a = 0.01 m2 the exterior air is at a (constant) p =1 atm and t = 27 c. the interior gas is 0.4 moles of (ideal) n2 and it has initial temperature 27.00 degrees c. 1. what is the initial pressure in the interior? a. 29.4 kpa b. 130.7 kpa c. 101.3 kpa the next three questions concern what happens when an amount of heat q is slowly added to the interior, raising the piston by 1 mm and raising the interior temperature to 27.40 c
Answers: 3

Physics, 22.06.2019 15:40, caveman171
Apotter's wheel moves uniformly from rest to an angular speed of 0.20 rev/s in 32.0 s. (a) find its angular acceleration in radians per second per second. rad/s2 (b) would doubling the angular acceleration during the given period have doubled final angular speed?
Answers: 1

Physics, 23.06.2019 00:00, kerena8291
You can estimate the degree to which a bond between two atoms is ionic or covalent by calculating the
Answers: 3
Do you know the correct answer?
Acylinder, which is in a horizontal position, contains an unknown noble gas at 4.00 × 10 4 pa 4.00×1...
Questions in other subjects:


Chemistry, 12.01.2020 15:31

History, 12.01.2020 15:31


English, 12.01.2020 15:31





Mathematics, 12.01.2020 15:31


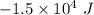

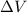 = Change in volume =
= Change in volume =  (negative because it is decreasing)
(negative because it is decreasing)