
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. sufficient pcl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250 °c. calculate the pressure of pcl5 after the system has reached equilibrium.
a. 1.50 atm
b. 1.24 atm
c. 4.24 atm
d. 0.94 atm
e. 1.12 atm

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 18:00, Zaydblackwood06
The photo shows sugar dissolved into a solution with excess sugar at the bottom of the jar this type of solution is classified as a. unsaturated b. compound c. saturated d. plasma
Answers: 1

Physics, 22.06.2019 18:00, ggdvj9gggsc
Atank is filled with an ideal gas at 400 k and pressure of 1.00 atm . part a the tank is heated until the pressure of the gas in the tank doubles. what is the temperature of the gas?
Answers: 3


Physics, 23.06.2019 02:30, yeehaw777
Atoms seldom exist as independent particles in nature a) as single particles, most atoms have low potential energy b) their electronegativity is much lower when they combine with other atoms c) atoms are more stable when they combine with other atoms d) neutral particles are rare
Answers: 2
Do you know the correct answer?
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. suffici...
Questions in other subjects:

History, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31


History, 08.11.2019 18:31

History, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31





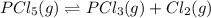
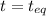 2.74-x x x
2.74-x x x
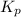 for the given reaction follows:
for the given reaction follows:
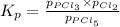
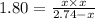
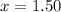
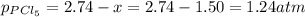
 at equilibrium is 1.24 atm
at equilibrium is 1.24 atm



