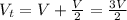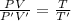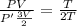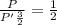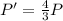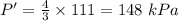Physics, 14.12.2019 06:31, sierraseideman1023
Asystem of ideal gas has an initial pressure of 111 kpa and occupies a volume of 5.00 liters. doubling the system’s absolute temperature by means of a constant-pressure process would require an amount of work w. instead, you decide to double the absolute temperature by carrying out two processes in sequence, a constant-pressure process followed by a constant-volume process. in this case, the total work done in the two-process sequence is w/2. calculate the final pressure of the system.

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 16:00, taylorbug6161
From the perspective of an employee that effective channeling of work related information and concerns
Answers: 1

Physics, 22.06.2019 16:30, Nerdylearner8639
Is there a point between a 10 nc charge and a 20 nc charge at which the electric field is zero?
Answers: 2

Physics, 22.06.2019 18:30, ochoanene822
Which of the following is not a means to accelerating? question 4 options: a)increase speed b)remain still c)decrease speed d)change direction
Answers: 2

Physics, 22.06.2019 21:50, gshreya2005
Which component is used to measure the current in a circuit? oa. switch ob. resistor oc. ammeter the answer is c. for y’all plato people
Answers: 1
Do you know the correct answer?
Asystem of ideal gas has an initial pressure of 111 kpa and occupies a volume of 5.00 liters. doubli...
Questions in other subjects:


Mathematics, 26.05.2021 02:40



Social Studies, 26.05.2021 02:40




Mathematics, 26.05.2021 02:40


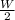
 (2)
(2)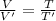
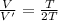
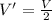
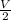 given by:
given by: