
Physics, 11.12.2019 20:31, akitchen10
At 2730 °c, hydrogen molecules dissociate into hydrogen atoms according to the equation h2 (g) → 2 h(g). 10.0 g h2 (g) is placed into a 100.0-l container, sealed and heated to 2730 °c so that the hydrogen molecules begin to dissociate. what is the partial pressure of hydrogen molecules and the total pressure within the container once 25% of h2 has dissociated?

Answers: 2
Other questions on the subject: Physics

Physics, 21.06.2019 19:30, sariyamcgregor66321
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies. molten iron has a density of 7.0g/cm cubed. in its solid state, iron has a density of 8.0g/cm cubed.
Answers: 3

Physics, 22.06.2019 06:30, bluevibe
2kg of refrigerant 134a undergoes a polytropic process in a piston-cylinder assembly from an initial state of saturated vapor at 2 bar to a final state of 12 bar, 80 degree c. a)determine the work for the process in kj. b)sketch the process on a p-v diagram.
Answers: 2

Do you know the correct answer?
At 2730 °c, hydrogen molecules dissociate into hydrogen atoms according to the equation h2 (g) → 2 h...
Questions in other subjects:

Social Studies, 17.07.2019 17:00



History, 17.07.2019 17:00

History, 17.07.2019 17:00

Biology, 17.07.2019 17:00

Social Studies, 17.07.2019 17:00



World Languages, 17.07.2019 17:00


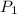 be the partial pressure of hydrogen atom
be the partial pressure of hydrogen atom



