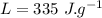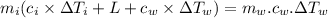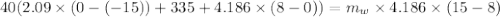A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) containing water at 15.00°c.
when equilibrium is reached, the final temperature is 8.00°c.
how much water did the calorimeter contain initially?
the specific heat of ice is 2090 j/kg • k, that of water is 4186 j/kg • k, and the latent heat of fusion of water is 33.5 × 104 j/kg.

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 13:30, markmoroney22
Would corn syrup, molasses, or pancake syrup make a good lubricant in a car engine? explain your answer.
Answers: 1

Physics, 21.06.2019 21:30, alexandraparava
In what direction does the medium move relative to the direction of the wave? explain.
Answers: 3

Physics, 22.06.2019 05:00, shimmerandshine1
At time t=0, a particle is located at the point (3,6,9). it travels in a straight line to the point (5,2,7), has speed 8 at (3,6,9) and constant acceleration 2i−4j−2k. find an equation for the position vector of the particle.
Answers: 2

Physics, 22.06.2019 19:30, leannehounschell
Acamcorder has a power rating of 20 watts. if the output voltage from its battery is 9 volts, what current does it use?
Answers: 2
Do you know the correct answer?
A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) contain...
Questions in other subjects:











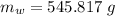
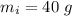 initial temperature of ice block,
initial temperature of ice block,  initial temperature of water,
initial temperature of water,  final temperature of mixture,
final temperature of mixture, 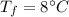 specific heat of ice,
specific heat of ice, 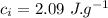 specific heat of water,
specific heat of water, 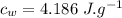 Latent heat of fusion of water,
Latent heat of fusion of water,