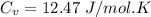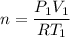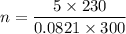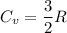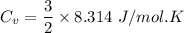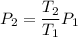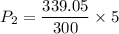Physics, 07.12.2019 03:31, ggpro4life3000
An ideal monatomic gas is contained in a vessel of constant volume 0.230 m3. the initial temperature and pressure of the gas are 300 k and 5.00 atm, respectively. the goal of this problem is to find the temperature and pressure of the gas after 23.0 kj of thermal energy is supplied to the gas.
(a) use the ideal gas law and initial conditions to calculate the number of moles of gas in the vessel.
mol
(b) find the specific heat of the gas.
j/k
(c) what is the work done by the gas during this process?
kj
(d) use the first law of thermodynamics to find the change in internal energy of the gas.
kj
(e) find the change in temperature of the gas.
k
(f) calculate the final temperature of the gas.
k
(g) use the ideal gas expression to find the final pressure of the gas.
atm

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 21:20, janayshas84
An artificial satellite circles the earth in a circular orbit at a location where the acceleration due to gravity is 6.03 m/s^2. determine the orbital period of the satellite.
Answers: 3


Do you know the correct answer?
An ideal monatomic gas is contained in a vessel of constant volume 0.230 m3. the initial temperature...
Questions in other subjects:

English, 19.07.2021 19:00

Mathematics, 19.07.2021 19:10