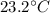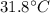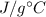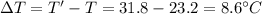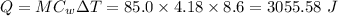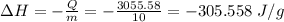In a coffee-cup calorimeter experiment, 10.00 g of a soluble ionic compound was added to the calorimeter contained 75.0 g h2o initially at 23.2°c. the final temperature of the solution was 31.8°c. what was the change in enthalpy for the dissolution of this compound?

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 00:30, dondre54
During spring semester at mit, residents of the parallel buildings of the east campus dorms battle one another with large catapults that are made with surgical hose mounted on a window frame. a balloon filled with dyed water is placed in a pouch attached to the hose, which is then stretched through the width of the room. assume that the stretching of the hose obeys hooke's law with a spring constant of 89.0 n/m. if the hose is stretched by 5.80 m and then released, how much work does the force from the hose do on the balloon in the pouch by the time the hose reaches its relaxed length? unitst 3 number-1497 the tolerance is +/-5% open show work click if you would like to show work for this question:
Answers: 2

Physics, 22.06.2019 10:10, princeofpowerjr
Which branches of natural science include the study of an organism that lived 10 million years ago
Answers: 1

Physics, 22.06.2019 19:00, maddied2443
Matter that emits no light at any wavelength is called
Answers: 2
Do you know the correct answer?
In a coffee-cup calorimeter experiment, 10.00 g of a soluble ionic compound was added to the calorim...
Questions in other subjects: