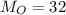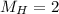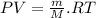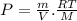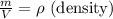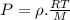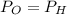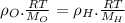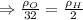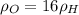Physics, 28.11.2019 05:31, thomasmurphy200
Two rigid tanks of equal size and shape are filled with different gases. the tank on the left contains oxygen, and the tank on the right contains hydrogen. assume both gases are ideal. the molar masses of oxygen and hydrogen are 32 and 2, respectively. both containers are at the same temperature. a pressure gauge is pin oxygen hydrogen connected to each tank. both gauges show a reading of 230 kpa.
is the number of oxygen molecules in the left container greater than, less than, or equal to the number of hydrogen molecules in the right container? explain your reasoning.

Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 19:30, ggdvj9gggsc
A500g object falls off a cliff and losers 100 j from its gravitational potential energy store. if the gravitational field strength g=9.8n/kg, how high is the cliff?
Answers: 1


Physics, 22.06.2019 11:30, joThompson
4. a 75.0 g piece of ag metal is heated to and dropped into 50.0 g of water at the final temperature of the mixture is what is the specific heat capacity of silver? 5. a 465 g chunk of iron is removed from petrucci, ralph h.. general chemistry (p. 290). pearson education. kindle edition.
Answers: 3

Physics, 22.06.2019 16:00, alecnewman2002
The energy produced as a result of this flow of electrons from atom to atom is called
Answers: 3
Do you know the correct answer?
Two rigid tanks of equal size and shape are filled with different gases. the tank on the left contai...
Questions in other subjects:

English, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01


Health, 16.10.2020 17:01





Biology, 16.10.2020 17:01