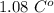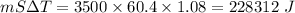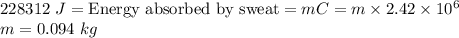Answers: 2
Other questions on the subject: Physics

Physics, 21.06.2019 23:30, xxxamslashxxx9
If 21.2 kcals of energy is released by this reaction, how many kj does the reaction release? (1 cal 4.184 j)
Answers: 1

Physics, 22.06.2019 04:00, tamyahamlin02p6b7yt
Determine the maximum r-value of the polar equation r =3+3 cos 0
Answers: 3

Do you know the correct answer?
The latent heat of vaporization of h₂o at body temperature (37°c) is 2.42 x 10⁶ j/kg. to cool the bo...
Questions in other subjects:











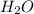 at 37°C is
at 37°C is 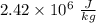 .
.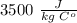 . Jogger weights 60.4 kg. Body temperature decreases by
. Jogger weights 60.4 kg. Body temperature decreases by