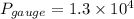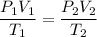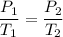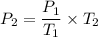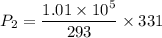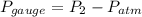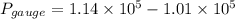Suppose a gas-filled incandescent light bulb is manufactured to have atmospheric pressure in it at 20.0°c. find the gauge pressure inside such a bulb when it is hot, assuming its average temperature is 58.0°c and neglecting any change in volume due to thermal expansion or gas leaks.

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 00:30, katiebug197
Orange juice has a lower or higher viscosity than chocolate syrup
Answers: 2

Physics, 22.06.2019 07:50, dimondqueen511
Calculate the ratio of h+ ions to oh– ions at a ph = 6. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 6. are they the same? why or why not? record your explanation in table a. what is the concentration of h+ ions at a ph = 6? mol/l what is the concentration of oh– ions at a ph = 6? mol/l what is the ratio of h+ ions to oh– ions at a ph = 6? : 1
Answers: 1

Physics, 22.06.2019 15:50, janeou17xn
Decreased sensitivity to an unchanging stimulus is known as
Answers: 3
Do you know the correct answer?
Suppose a gas-filled incandescent light bulb is manufactured to have atmospheric pressure in it at 2...
Questions in other subjects:




Health, 13.12.2019 04:31