
Physics, 14.11.2019 01:31, alexwlodko
What happens to the reaction 2 n o 2 in equilibrium with n 2 o 4 plus 57.3 kilojoules when the temperature of the reaction is increased?
more product forms
more reactant forms
the reaction stops
no change occurs

Answers: 1
Other questions on the subject: Physics


Physics, 22.06.2019 00:10, oktacos
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 11:30, tommyaberman
You've already seen the value of 9.8 in this lesson. what's this value called? what quantity does it represent?
Answers: 2
Do you know the correct answer?
What happens to the reaction 2 n o 2 in equilibrium with n 2 o 4 plus 57.3 kilojoules when the tempe...
Questions in other subjects:

Biology, 20.04.2020 07:16


English, 20.04.2020 07:16

Mathematics, 20.04.2020 07:16






Mathematics, 20.04.2020 07:16


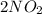 ⇒
⇒ per mole
per mole



