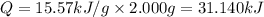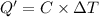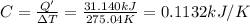Physics, 11.11.2019 20:31, clickbaitdxl
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe and measuring the change in temperature. for example, the combustion energy of glucose is 15.57 kj/g. when a 2.000 g sample of glucose burns in a constant volume calorimeter, the calorimeter temperature increases from 21.45 to 23.34°c. find the total heat capacity of the calorimeter (in kj/k).

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 06:30, kdfawesome5582
Apebble is thrown into a calm lake, ripples are formed from the center and move outward. the water particles in the lake travel in a circular pattern that moves up and down on the surface of a lake, and the energy travels a) diagonally. b) downward. c) horizontally. d) upward.
Answers: 2

Physics, 22.06.2019 06:30, itzyoboyCj
Organelles are 1. responsible for producing power for the cell 2. tiny structures in the cell that carry out the cell's activities 3. responsible for digestion in the cell 4. found outside of the membrane
Answers: 1

Physics, 22.06.2019 20:30, justaguy15
Atypical jetliner lands at a speed of 146 mi/h and decelerates at the rate of (10.4 mi/h)/s. if the jetliner travels at a constant speed of 146 mi/h for 1.5 s after landing before applying the brakes, what is the total displacement of the jetliner between touchdown on the runway and coming to rest?
Answers: 2

Physics, 23.06.2019 00:30, janeou17xn
What os the equation of the line described below written in slope-intercept form? the line passing through point (0,0) and parallel to the line whose equation is 3x+2y-6=0
Answers: 3
Do you know the correct answer?
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe a...
Questions in other subjects:


Mathematics, 29.10.2019 01:31



Business, 29.10.2019 01:31

Arts, 29.10.2019 01:31


History, 29.10.2019 01:31