
Physics, 08.11.2019 03:31, goldwinner300
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe and measuring the change in temperature. for example, the combustion energy of glucose is 15.57 kj/g. when a 1.500 g sample of glucose burns in a constant volume calorimeter, the calorimeter temperature increases from 21.45 to 23.34°c. find the total heat capacity of the calorimeter (in kj/k).

Answers: 2
Other questions on the subject: Physics

Physics, 21.06.2019 19:30, yairreyes01
Is it possible to use the energy from two atoms colliding to create a clean burning lasting fuel source with a large amount of force? is it also possible to harness that energy from the two atoms colliding to create a clean energy source that will last for years from just two atoms colliding. is there a possibility to control and store that energy for years but slowly let the energy out enough to power cities cheaper and faster.
Answers: 3


Physics, 22.06.2019 06:20, walmartislife
Part 1: a magnetic levitation or maglev train rides rails without touching them. explain how this works using your data. include the appropriate magnet drawing in your answer. part 2: two objects are near a bar magnet. one is about 1 cm away, while the other is 6 cm away. compare and contrast the magnetic force that affects each object. use your data to answer the question
Answers: 1

Physics, 22.06.2019 15:30, jjjjjj4999
Match each scenario to the form of energy it represents
Answers: 2
Do you know the correct answer?
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe a...
Questions in other subjects:


Physics, 12.11.2020 04:00

Mathematics, 12.11.2020 04:00

Spanish, 12.11.2020 04:00

Mathematics, 12.11.2020 04:00




Mathematics, 12.11.2020 04:00

History, 12.11.2020 04:00


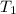 = 21.45
= 21.45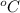
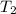 = 23.34
= 23.34




