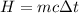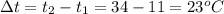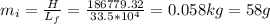Physics, 31.10.2019 01:31, niasheffield65
The temperature of 1.94 kg of water is 34 °c. to cool the water, ice at 0°c is added to it. the desired final temperature of the water is 11 °c. the latent heat of fusion for water is 33.5 × 10^4 j/kg, and the specific heat capacity of water is 4186 j/(kg·c°). ignoring the container and any heat lost or gained to or from the surroundings, determine how much mass m of ice should be added?

Answers: 3
Other questions on the subject: Physics


Physics, 22.06.2019 09:10, kingdesto3481
Which lists the organs in the correct order as food passes from the mouth to anus?
Answers: 1

Physics, 22.06.2019 18:30, yclark98563p5z2gt
Suppose you plot the distance traveled by an object at various times and you discover that the graph is not a straight line. what does this indicate about the object's acceleration?
Answers: 3
Do you know the correct answer?
The temperature of 1.94 kg of water is 34 °c. to cool the water, ice at 0°c is added to it. the desi...
Questions in other subjects:

Mathematics, 09.04.2020 02:52

English, 09.04.2020 02:52

Engineering, 09.04.2020 02:52


Mathematics, 09.04.2020 02:52

Mathematics, 09.04.2020 02:52


Biology, 09.04.2020 02:52

Mathematics, 09.04.2020 02:52