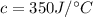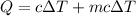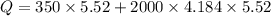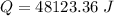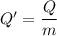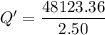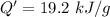Physics, 17.10.2019 20:20, awesomegrill
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimeter" with a heat capacity (excluding water) of 350 j/°c and which contained 2.00 liters of water (density = 1.00 g/ml and specific heat capacity = 4.184 j/°c•g), the resulting temperature change was measured to be 5.52°c. calculate the thermal energy (in kj) released per gram of hydrocarbon combusted. (1) 48.1 kj/g (2) 0.773 kj/g (3) 19.2 kj/g (4) 18.5 kj/g (5) 46.2 kj/g

Answers: 2
Other questions on the subject: Physics

Physics, 21.06.2019 20:00, desscraft30
Which of the following represents an upright image? a. -do b. +m c. -m d. +do
Answers: 1


Do you know the correct answer?
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimet...
Questions in other subjects:



Mathematics, 03.02.2022 14:00



English, 03.02.2022 14:00

Chemistry, 03.02.2022 14:00

Mathematics, 03.02.2022 14:00

English, 03.02.2022 14:00