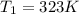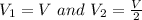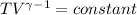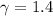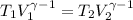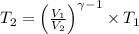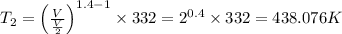Avertical cylinder is divided into two parts by a movable piston of mass m. the piston and cylinder system is well insulated (that is, no heat can flow in or out of the system) and the piston is initially held at rest. the top part of the cylinder is evacuated and the bottom part is filled with 1.00 mole of diatomic ideal gas at temperature 332 k. after the piston is released and the system comes to equilibrium, the volume occupied by gas is halved. find the final temperature of the gas.

Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 15:00, charleetrill8304
If you were on a jury, do you think you would expect individual characteristics in the evidence? why or why not? what effects might it have if individuals expect to have individuals characteristics presented? this question is for forensic science
Answers: 2

Physics, 22.06.2019 10:00, nayellisoto15
Asap and show ! a 14 kg rock starting from rest free falls through a distance of 5.0 m with no air resistance. find the momentum change of the rock caused by its fall and the resulting change in the magnitude of earths velocity. earth mass is 6.0 * 10^24 kg. show your work assuming the rock earth system is closed.
Answers: 2

Physics, 22.06.2019 12:30, 21brooklynmartin
Which levels of government establish and implement educational requirements for minors
Answers: 1

Physics, 22.06.2019 14:50, MoogleCaliS
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2
Do you know the correct answer?
Avertical cylinder is divided into two parts by a movable piston of mass m. the piston and cylinder...
Questions in other subjects:








History, 18.07.2019 00:10