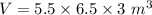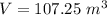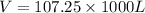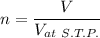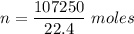Physics, 09.10.2019 02:20, mullery7482
Th e heat capacity of air is much smaller than that of water, and relatively modest amounts of heat are needed to change its temperature. th is is one of the reasons why desert regions, although very hot during the day, are bitterly cold at night. th e heat capacity of air at room temperature and pressure is approximately 21 j k−1 mol−1. how much energy is required to raise the temperature of a room of dimensions 5.5 m × 6.5 m × 3.0 m by 10°c? if losses are neglected, how long will it take a heater rated at 1.5 kw to achieve that increase given that 1 w = 1 j s−1?

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 04:50, badpotterchris
Find v(t), given acceleration a(t)=7j and initial velocity v(0)=k
Answers: 2


Physics, 22.06.2019 17:30, funnybugy16
How does the entropy of steam compare to the entropy of ice?
Answers: 2

Physics, 22.06.2019 19:20, gokusupersaiyan12345
The dipole moment of the water molecule (h2o) is 6.17x10^-30 c. m. consider a water molecule located at the origin whose dipole moment p points in the +x-direction. a chlorine ion ( of charge-1.60x10^-19c , is located at x=3.00x10^-9m . assume that is much larger than the separation d between the charges in the dipole, so that the approximate expression for the electric field along the dipole axis can be used. a) find the magnitude of the electric force that the water molecule exerts on the chlorine ion. b) what is the direction of the electric force. -x-direction or +x-direction c) is this force attractive or repulsive?
Answers: 1
Do you know the correct answer?
Th e heat capacity of air is much smaller than that of water, and relatively modest amounts of heat...
Questions in other subjects:

Computers and Technology, 06.07.2020 23:01



Mathematics, 06.07.2020 23:01

English, 06.07.2020 23:01

Mathematics, 06.07.2020 23:01

Mathematics, 06.07.2020 23:01


Mathematics, 06.07.2020 23:01