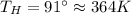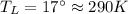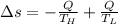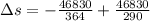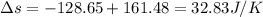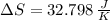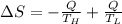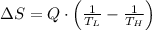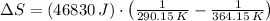Physics, 30.09.2019 23:30, noellelovebug1214
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one at a temperature of 17.0 °c. these reservoirs are brought into thermal contact long enough for 46830 j of heat to flow from the hot water to the cold water. assume that the reservoirs are large enough so that the temperatures do not change significantly. what is the total change in entropy resulting from this heat exchange between the hot water and the cold water?

Answers: 3
Similar questions



Do you know the correct answer?
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one a...
Questions in other subjects:

Mathematics, 29.10.2020 20:10







English, 29.10.2020 20:10