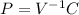When dealing with 1 liter of argon as a closed system and as an ideal gas that undergoes an isothermal expansion, which of the following expressions can be used to describe the state of your thermodynamic system? group of answer choices a. pv = r. b. pv = const. c. p is inverse proportional to v. d. answers (a) and (b) are both correct and they give the same information about the system under investigation. e. answers (b) and (c) are both correct and they give the same information about the system under investigation.

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 10:10, Fionaauggies
How will the system respond to a temperature increase?
Answers: 1

Physics, 22.06.2019 16:00, Christyy1837
What part of the ear is names after tools, such as the hammer and the anvil?
Answers: 1

Physics, 22.06.2019 19:00, sanchez626
Friction removes energy from objects in motion. which statement best describes how this works? a) friction transforms ke into thermal energy b) friction transfers thermal energy to ke c) friction transforms te into pe d) friction transforms pe into ke e) friction transfers ke into pe
Answers: 1

Physics, 23.06.2019 00:50, Madisonaubrey22
A980-kg sports car collides into the rear end of a 2300-kg suv stopped at a red light. the bumpers lock, the brakes are locked, and the two cars skid forward 2.6m before stopping. the police officer, estimating the coefficient of kinetic friction between tires and road to be 0.80, calculates the speed of the sports car at impact. what was that speed?
Answers: 3
Do you know the correct answer?
When dealing with 1 liter of argon as a closed system and as an ideal gas that undergoes an isotherm...
Questions in other subjects:

Social Studies, 22.07.2019 08:30


Mathematics, 22.07.2019 08:30


Mathematics, 22.07.2019 08:30

Mathematics, 22.07.2019 08:30



Mathematics, 22.07.2019 08:30