
Physics, 24.09.2019 05:00, lexybellx3
Suppose 16.0 g of oxygen (o2) is heated at constant atmospheric pressure from 26.4°c to 111°c. (a) how many moles of oxygen are present? (take the molar mass of oxygen to be 32.0 g/mol) (b) how much energy is transferred to the oxygen as heat? (the molecules rotate but do not oscillate.) (c) what fraction of the heat is used to raise the internal energy of the oxygen?

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 22:20, kl8774
Aboxcar traveling at 12 m/s approaches a string of 5 identical boxcars sitting stationary on the track. the moving boxcar collides and links with the stationary cars and they all move off together along the track. what is the final speed of the cars immediately after the collision? (you may take the mass of each boxcar to be 18,537 kg.)
Answers: 1

Physics, 22.06.2019 06:30, 1234fatimasm
What are similarities and differences between refraction, reflection, diffraction and absorption?
Answers: 3

Physics, 22.06.2019 10:00, he0gaubong
Which atomic model was proposed as a result of j. j. thomson’s work?
Answers: 1

Do you know the correct answer?
Suppose 16.0 g of oxygen (o2) is heated at constant atmospheric pressure from 26.4°c to 111°c. (a) h...
Questions in other subjects:


Mathematics, 23.12.2020 02:00

English, 23.12.2020 02:00




Chemistry, 23.12.2020 02:00

Mathematics, 23.12.2020 02:00

English, 23.12.2020 02:00

Social Studies, 23.12.2020 02:00


 ----1
----1
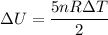 --------2
--------2



