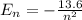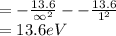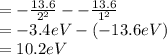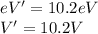Physics, 21.09.2019 04:30, juliaduenkelsbu
Calculate the the wavelength of the first balmer series of hydrogen is 6562 following: a) the ionization potential, and b) the first excitation potential of the hydrogen atom.

Answers: 1
Other questions on the subject: Physics


Physics, 21.06.2019 23:20, pineapplepizaaaaa
Acharge of 8.4x10^-4 c moves at an angle of 35 degrees to a magnetic field that has a field strength of 6.7x10^-3 t. if the magnetic force is 3.5 x10 ^-2 n, how fast is the charge moving?
Answers: 1


Physics, 22.06.2019 12:50, shollydot1379
Assume you measured the mass of the cart to be (500 ± 1) g and the mass of the additional mass you put on the cart to be (500 ± 1) g as well. since the scale you are using in the lab cannot measure objects heavier than 600g you will have to sum up individual pieces and propagate the error. so what would be the mass and the standard error of the cart and the mass
Answers: 3
Do you know the correct answer?
Calculate the the wavelength of the first balmer series of hydrogen is 6562 following: a) the ioniz...
Questions in other subjects:

Mathematics, 12.10.2019 23:30