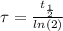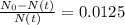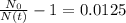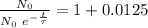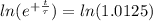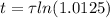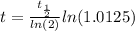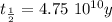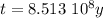The ages of rocks that contain fossils can be determined using the isotope 87rb. this isotope of rubidium undergoes beta decay with a half‑life of 4.75×1010y . ancient samples contain a ratio of 87sr to rb87 of 0.0125. given that 87sr is a stable product of the beta decay of 87rb, and assuming there was originally no 87sr present in the rocks, calculate the age of the rock sample. assume that the decay rate is constant over the relatively short lifetime of the rock compared to the half-life of 87rb.

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 18:10, Alexmills6093
Keneila is attempting to ski down a 20 m high friction free hill for the first time. she has a speed 10 m/s at the top. what is her kinetic energy when she is a the bottom, 20 m ? (a)2500 j (b)9800 j (c)12300 j (d)3100j (e)15000j
Answers: 2

Physics, 22.06.2019 07:40, Alex9089435028
Astudent creates a model of a closed ecosystem by filling a glass tank half full with water, then adding 10 snails and two small aquatic plants. the next day, all the snails are dead. what is the most likely cause of their death?
Answers: 3


Do you know the correct answer?
The ages of rocks that contain fossils can be determined using the isotope 87rb. this isotope of rub...
Questions in other subjects:

Geography, 06.06.2020 01:01



Mathematics, 06.06.2020 01:01




Chemistry, 06.06.2020 01:01


Mathematics, 06.06.2020 01:01


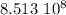 years
years 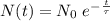
 is the initial quantity of the material, and
is the initial quantity of the material, and  is the mean lifetime of the material.
is the mean lifetime of the material.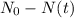
 ) by the relationship
) by the relationship