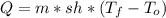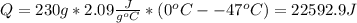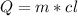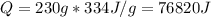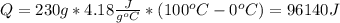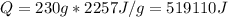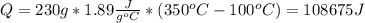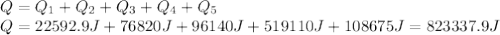Physics, 17.09.2019 23:20, 7thaohstudent
Water has the following thermodynamic constants: (1) specific heat liquid = 4.18 j/g °c, solid = 2.09 j/g °c, gas = 1.89 j/g °c, (2) heat of fusion = 334 j/g, and (3) heat of vaporization = 2257 j/g. for a sample of water at 1.0 atm of pressure, mass = 230 g at an initial temperature of -47 °c and a final temperature of 350 °c, answer the following questions: (1) how much heat is required to warm the solid sample to its melting point? j (2) how much heat is required to melt the sample? j (3) how much heat is required to warm the liquid sample to its boiling point? j (4) how much heat is required to vaporize the sample? j (5) how much heat is required to warm the gaseous sample to its final temperature? j and finally, (6) how much heat is required for the entire process to occur

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 01:40, janeou17xn
Crowbar of 5 metre is used to lift an object of 800 metre if the effort arm is 200cm calculate the force applied
Answers: 1

Physics, 22.06.2019 15:30, ayoismeisalex
What is a view of science and psychology that says the value of knowledge depends on its usefulness? a. pragmatism b. psychotherapy c. physiology
Answers: 2


Physics, 23.06.2019 06:30, Roninsongrant
What surface does the epithelial tissue cover? a. the surface of the body b. lines internal organs c. forms many glands d. all of the above
Answers: 3
Do you know the correct answer?
Water has the following thermodynamic constants: (1) specific heat liquid = 4.18 j/g °c, solid = 2....
Questions in other subjects:

Mathematics, 14.06.2020 22:57


Mathematics, 14.06.2020 22:57

Geography, 14.06.2020 22:57

History, 14.06.2020 22:57

Mathematics, 14.06.2020 22:57

Social Studies, 14.06.2020 22:57


History, 14.06.2020 22:57

Mathematics, 14.06.2020 22:57