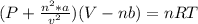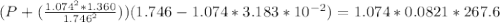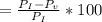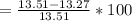Physics, 05.09.2019 16:10, skgoldsmith
According to the ideal gas law, a 1.074 mol sample of oxygen gas in a 1.746 l container at 267.6 k should exert a pressure of 13.51 atm. what is the percent difference between the pressure calculated using the van der waals' equation and the ideal pressure? for o2 gas, a = 1.360 l2atm/mol2 and b = 3.183×10-2 l/mol.

Answers: 1
Other questions on the subject: Physics


Physics, 22.06.2019 20:10, kerarucker12pe384k
Atruck with 34-in.-diameter wheels is traveling at 55 mi/h. find the angular speed of the wheels in rad/min, *hint convert miles to inches & hours to minutes:
Answers: 2

Physics, 23.06.2019 02:00, em387p3s1zr
Athird point charge q3 is now positioned halfway between q1 and q2. the net force on q2 now has a magnitude of f2,net = 5.861 n and points away from q1 and q3. what is the value (sign and magnitude) of the charge q3?
Answers: 2

Physics, 23.06.2019 02:00, Rosemckinney2351
Determine the time it takes for a satellite to orbit the earth in a circular "near-earth" orbit. the definition of "near-earth" orbit is one which is at a height above the surface of the earth which is small compared to the radius of the earth, so that you may take the acceleration due to gravity as essentially the same as that on the surface. does your result depend on the mass of the satellite?
Answers: 3
Do you know the correct answer?
According to the ideal gas law, a 1.074 mol sample of oxygen gas in a 1.746 l container at 267.6 k s...
Questions in other subjects:



Mathematics, 05.02.2021 06:30


Spanish, 05.02.2021 06:30


English, 05.02.2021 06:30



Mathematics, 05.02.2021 06:30