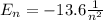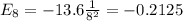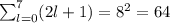Consider a hydrogen atom with the electron in the n 8 shell. what is the energy of this system? (the magnitude of the ground state energy of the hydrogen atom is 13.6 ev.) submit answer tries 0/20 how many subshells are in this shell? submit answer tries 0/20 how many electron orbits are in this main shell? submit answer tries 0/20 how many electrons would fit in this main shell?

Answers: 1
Similar questions

Physics, 04.08.2019 00:00, KpopSushi
Answers: 2

Physics, 21.08.2019 01:30, eden1017
Answers: 1

Physics, 21.08.2019 16:20, cheating53
Answers: 3
Do you know the correct answer?
Consider a hydrogen atom with the electron in the n 8 shell. what is the energy of this system? (th...
Questions in other subjects:

History, 20.04.2021 21:30

Mathematics, 20.04.2021 21:30

Mathematics, 20.04.2021 21:30




Computers and Technology, 20.04.2021 21:30

English, 20.04.2021 21:30

Mathematics, 20.04.2021 21:30

SAT, 20.04.2021 21:30