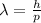Answers: 1
Similar questions

Physics, 27.06.2019 08:20, TrueKing184
Answers: 3


Physics, 23.07.2019 06:00, LoserMcBadface
Answers: 1

Physics, 07.09.2019 00:30, zionboy77
Answers: 3
Do you know the correct answer?
According to the de broglie relation, the wavelength of a "matter" wave is inversely proportional to...
Questions in other subjects:

Mathematics, 25.05.2021 23:40

Mathematics, 25.05.2021 23:40

Mathematics, 25.05.2021 23:40





Physics, 25.05.2021 23:40


World Languages, 25.05.2021 23:40