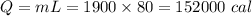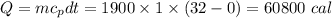Physics, 06.08.2019 04:10, tdahna0403
Water's heat of fusion is 80. cal/g , and its specific heat is 1.0calg⋅∘c . some velomobile seats have been designed to hold ice packs inside their cushions. if you started a ride with ice packs that held 1900 g of frozen water at 0 ∘c , and the temperature of the water at the end of the ride was 32 ∘c , how many calories of heat energy were absorbed?

Answers: 2
Similar questions

Chemistry, 01.07.2019 20:00, tlecuyer
Answers: 1

Physics, 22.07.2019 11:30, mm016281
Answers: 1

Chemistry, 02.09.2019 18:20, realmofgrads23
Answers: 3

Chemistry, 26.10.2019 20:43, jholbrook7643
Answers: 1
Do you know the correct answer?
Water's heat of fusion is 80. cal/g , and its specific heat is 1.0calg⋅∘c . some velomobile seats ha...
Questions in other subjects:


History, 22.02.2021 03:50

English, 22.02.2021 03:50

Mathematics, 22.02.2021 03:50