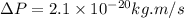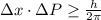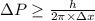Rutherford's scattering experiments gave the first indications that an atom consists of a small, dense, positively charged nucleus surrounded by negatively charged electrons. his experiments also allowed for a rough determination of the size of the nucleus. in this problem, you will use the uncertainty principle to get a rough idea of the kinetic energy of a particle inside the nucleus. consider a nucleus with a diameter of roughly 5.0×10^−15 meters. consider a particle inside the nucleus. the uncertainty δx in its position is equal to the diameter of the nucleus. what is the uncertainty δp of its momentum? to find this, use δx δp ≥ h

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 00:30, dondre54
During spring semester at mit, residents of the parallel buildings of the east campus dorms battle one another with large catapults that are made with surgical hose mounted on a window frame. a balloon filled with dyed water is placed in a pouch attached to the hose, which is then stretched through the width of the room. assume that the stretching of the hose obeys hooke's law with a spring constant of 89.0 n/m. if the hose is stretched by 5.80 m and then released, how much work does the force from the hose do on the balloon in the pouch by the time the hose reaches its relaxed length? unitst 3 number-1497 the tolerance is +/-5% open show work click if you would like to show work for this question:
Answers: 2


Physics, 22.06.2019 07:30, michaireid04
Identify the theory that can be used to explain each phenomenon. answers diffraction: wave theory interference: wave theory reflection: both particle and wave theories refraction: both particle and wave theories
Answers: 3

Physics, 22.06.2019 11:30, joThompson
4. a 75.0 g piece of ag metal is heated to and dropped into 50.0 g of water at the final temperature of the mixture is what is the specific heat capacity of silver? 5. a 465 g chunk of iron is removed from petrucci, ralph h.. general chemistry (p. 290). pearson education. kindle edition.
Answers: 3
Do you know the correct answer?
Rutherford's scattering experiments gave the first indications that an atom consists of a small, den...
Questions in other subjects:







History, 22.11.2019 06:31