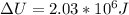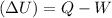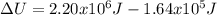Physics, 03.08.2019 06:10, leoiscoolcool
When water is boiled under a pressure of 2.00 atm, the heat of vaporization is 2.20×10^6 j/kg and the boiling point is 120 c. at this pressure, 1.00 kg of water has a volume of 1.00×10^−3 m3, and 1.00 kg of steam has a volume of 0.824 m^3.(a)compute the work done when 1.00kg of steam is formed at this temperature(b)compute the increase in internal energy of the water.

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 09:00, bendmads04
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1

Physics, 23.06.2019 02:00, sjjarvis4806
Ideal meters problem. what internal resistance is ideal for a voltmeter? what internal resistance is ideal for an ammeter?
Answers: 2

Physics, 23.06.2019 07:00, school7067
Which best offense of relationship between speed and velocity speed is velocity what displacement velocity and speed with displacement spee is based on a specific direction velocity isis based on displacement
Answers: 1
Do you know the correct answer?
When water is boiled under a pressure of 2.00 atm, the heat of vaporization is 2.20×10^6 j/kg and th...
Questions in other subjects:




Mathematics, 05.07.2019 10:00

Mathematics, 05.07.2019 10:00

Business, 05.07.2019 10:00

Business, 05.07.2019 10:00

Mathematics, 05.07.2019 10:00


Mathematics, 05.07.2019 10:00