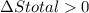Ahot object and a cold object are placed in thermal contact and the combination is isolated. they transfer energy until they reach a common temperature. the change δsh in the entropy of the hot object, the change δsc in the entropy of the cold object, and the change δstotal in the entropy of the combination are:
a) δsh > 0, δsc > 0, δstotal > 0 b) δsh < 0, δsc > 0, δstotal > 0 c) δsh < 0, δsc > 0, δstotal < 0 d) δsh > 0, δsc < 0, δstotal > 0 e) δsh > 0, δsc < 0, δstotal < 0

Answers: 3
Similar questions

Physics, 02.07.2019 18:00, dej33
Answers: 1

Chemistry, 04.08.2019 06:30, iidoqeii16ovh8tk
Answers: 2

Physics, 20.08.2019 22:00, 083055
Answers: 1

Physics, 13.09.2019 17:20, CutiePie8960
Answers: 3
Do you know the correct answer?
Ahot object and a cold object are placed in thermal contact and the combination is isolated. they tr...
Questions in other subjects:

History, 30.07.2020 14:01






Chemistry, 30.07.2020 14:01


Mathematics, 30.07.2020 14:01


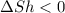 ,
, 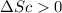 ,
,