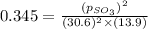Physics, 25.07.2019 05:20, diamondk2019
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2(g)+o2(g)→2so3(g) at equilibrium, the partial pressure of so2 is 30.6 atm and that of o2 is 13.9 atm. the partial pressure of so3 is atm.

Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 13:30, hayleighsmall111
This is how zirconium appears in the periodic table. rounded to the nearest whole number, how many electrons are in an atom of zirconium?
Answers: 2

Physics, 22.06.2019 07:00, shaffergabe10
Oxygen and hydrogen gas are at the same temperature t. what is the ratio of kinetic energies of oxygen molecule and hydrogen molecule if oxygen is 16 times heavier than hydrogen.
Answers: 3


Physics, 22.06.2019 16:20, nessuhbae6722
What is the single most important equation in all of physics?
Answers: 1
Do you know the correct answer?
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2(g)+o2(g)→2so3(g)...
Questions in other subjects:

Advanced Placement (AP), 23.10.2019 07:00

English, 23.10.2019 07:00

Biology, 23.10.2019 07:00


Health, 23.10.2019 07:00

Social Studies, 23.10.2019 07:00


English, 23.10.2019 07:00



 is, 67.009 atm
is, 67.009 atm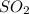 at equilibrium = 30.6 atm
at equilibrium = 30.6 atm at equilibrium = 13.9 atm
at equilibrium = 13.9 atm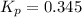

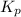 will be,
will be,