
A31.15−g stainless steel ball bearing at 103.27°c is placed in a constant-pressure calorimeter containing 110.3 g of water at 22.59°c. if the specific heat of the ball bearing is 0.474 j / (g · °c), calculate the final temperature of both the water and steel when they equilibrate. assume the calorimeter to have negligible heat capacity.

Answers: 1
Other questions on the subject: Physics


Physics, 23.06.2019 00:30, asher456581
Diego kicks a soccer ball from the end line. his fellow students time and mark the soccer ball as it moves down the field. the graph represents the ball's progress. based on the graph, during what time is the velocity constant and positive?
Answers: 1

Physics, 23.06.2019 00:30, sarahsteelman
Define the terms moral development and moral reasoning.
Answers: 1

Do you know the correct answer?
A31.15−g stainless steel ball bearing at 103.27°c is placed in a constant-pressure calorimeter conta...
Questions in other subjects:


Chemistry, 19.05.2021 20:40

Mathematics, 19.05.2021 20:40

Mathematics, 19.05.2021 20:40

Mathematics, 19.05.2021 20:40


Mathematics, 19.05.2021 20:40

Biology, 19.05.2021 20:40


Mathematics, 19.05.2021 20:40


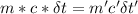
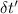 = change in temperature of water = t-22.59
= change in temperature of water = t-22.59



