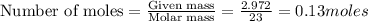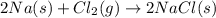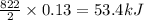Physics, 18.07.2019 21:10, cschellfamily
The reaction between sodium metal and chlorine gas produces 822 kj of heat energy for every chlorine molecule consumed [2 na(s) + cl2(g) → 2 nacl(s)]. how much heat is released (in kj) if 2.972 g of na are consumed in the reaction?

Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 18:30, mariatorres7
Which of these describe conduction transfer of heat between two objects that are touching transfer of heat by the actual movement of war matter the process in which energy is imitated by one of the object transmitted through space and absorbed by another a process in which energy is released by the molecules breaking apart
Answers: 1

Physics, 22.06.2019 06:00, Bengynease2598
What will a positive and a negative charge do if they are separated from each other?
Answers: 3

Do you know the correct answer?
The reaction between sodium metal and chlorine gas produces 822 kj of heat energy for every chlorine...
Questions in other subjects:


Mathematics, 05.03.2021 04:50

Mathematics, 05.03.2021 04:50

Mathematics, 05.03.2021 04:50

Health, 05.03.2021 04:50



English, 05.03.2021 04:50

Engineering, 05.03.2021 04:50


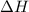 for the reaction comes out to be negative.
for the reaction comes out to be negative.