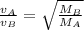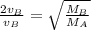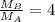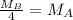Physics, 13.07.2019 01:40, jazzilove710
Consider two ideal gases, a & b, at the same temperature. the rms speed of the molecules of gas a is twice that of gas b. how does the molecular mass of a compare to that of b? (a) it is twice that of b. (b) it is one half that of b (c) it is four times that of b (d) it is one fourth that of b. (e) it is 1.4 times that of b.

Answers: 3
Similar questions

Chemistry, 23.06.2019 08:40, Riplilpeep
Answers: 1

Chemistry, 19.07.2019 19:20, k123loveme
Answers: 2

Chemistry, 28.08.2019 18:30, BreBreDoeCCx
Answers: 1
Do you know the correct answer?
Consider two ideal gases, a & b, at the same temperature. the rms speed of the molecules of gas...
Questions in other subjects:

Mathematics, 21.04.2021 20:10

Physics, 21.04.2021 20:10

Mathematics, 21.04.2021 20:10


Mathematics, 21.04.2021 20:10


Mathematics, 21.04.2021 20:10


Mathematics, 21.04.2021 20:10

Physics, 21.04.2021 20:10