
Physics, 03.07.2019 23:30, itaheart101
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorimeter at a temperature of 22°c. determine the final temperature of the system consisting of the ice, water, and calorimeter. remember that the ice must first warm to 0°c, melt, and then continue warming as water. the specific heat of ice is 0.500 cal/g·°c = 2090 j/kg°c.

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 04:00, katieleeisaacs8368
Ametal ball with a mass of 0.028 kg is dropped from rest at a height of 1.0 meters above the ground. assuming the energy in the ball is conserved, how much kinetic energy will the ball have when it is 0.5 meters above the ground?
Answers: 1

Physics, 22.06.2019 10:00, mathscience9301
Which fact supports the conclusion that there will be fewer farm managers in the future? a) a farmer manager’s duties vary by the type of farm. b) farm technology is replacing some administrative jobs c) farm managers often need experience but not education. d) administrative duties include budgeting and training staff.
Answers: 2


Physics, 23.06.2019 00:00, isabella4141
Which is an advantage of subdividing science into different areas?
Answers: 3
Do you know the correct answer?
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorime...
Questions in other subjects:





English, 06.05.2020 07:42

Mathematics, 06.05.2020 07:42

History, 06.05.2020 07:42

English, 06.05.2020 07:42



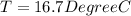
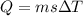
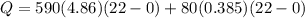
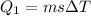
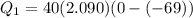
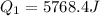
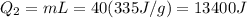
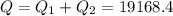
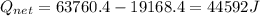
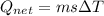
![44592 = (590 + 40)(4.186)(T - 0) + 80(0.385)(T - 0)[tex]T = 16.7 Degree C](/tpl/images/0048/1109/c6b32.png)




