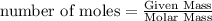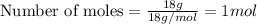Physics, 29.06.2019 22:00, jaidenkenna2001
Specific heat of water = 4.186 j g°c specific heat of ice = 2.00 j g°c molar heat of fusion = 6030 j mol molar heat of vaporization = 40790 j mol you take an ice cube (mass = 18g) from the freezer (t = -10°c) and place it on the table. later that day, you notice a puddle of water on the table that has reached ambient room temperature (20°c). how much heat must have been absorbed to make this happen?

Answers: 1
Similar questions



Chemistry, 14.11.2019 08:31, helpmewithmath70
Answers: 1
Do you know the correct answer?
Specific heat of water = 4.186 j g°c specific heat of ice = 2.00 j g°c molar heat of fusion = 6030...
Questions in other subjects:

English, 25.09.2020 14:01

History, 25.09.2020 14:01



History, 25.09.2020 14:01

Biology, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01

History, 25.09.2020 14:01

English, 25.09.2020 14:01