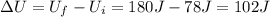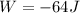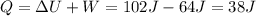Physics, 15.07.2019 09:00, bluetigerbird5323
Jin knows that the initial internal energy of a closed system is 78 j and the final internal energy is 180 j. he also knows that 64 j of energy are used to do work. to find the heat added to the system, jin completes the steps below. 1. add the initial internal energy plus the final internal energy to find the change in internal energy. 2. add the change in internal energy to the energy used to do work. 3. write the answer in joules.

Answers: 1
Other questions on the subject: Physics


Physics, 22.06.2019 13:00, chie42
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2


Physics, 22.06.2019 20:30, sza2016
This is a form of winter precipitation. it is frozen precipitation falling as ice pellets. snowflakes melt into raindrops as they pass through a thin layer of warmer air. the raindrops then refreeze into particles of ice when they fall into a layer of sub-freezing air near the surface of the earth. this precipitation is called a) hail. b) rain. c) sleet. d) snow.
Answers: 1
Do you know the correct answer?
Jin knows that the initial internal energy of a closed system is 78 j and the final internal energy...
Questions in other subjects:



Biology, 02.07.2019 00:30

History, 02.07.2019 00:30



Mathematics, 02.07.2019 00:30

Biology, 02.07.2019 00:30

Physics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30


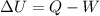 (1)
(1)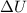 is the variation of internal energy
is the variation of internal energy