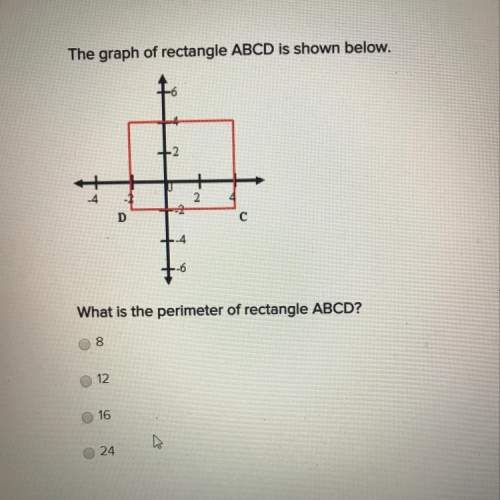Mathematics, 17.05.2021 16:30, jennelledenise
Un elemento tiene las siguientes abundancias naturales y masas isotópicas: 90,92% de abundancia con 19,99 amu, 0,26% de abundancia con 20,99 amu y 8,82% de abundancia con 21,99 amu. Cuál es su la masa atómica promedio de este elemento

Answers: 1
Other questions on the subject: Mathematics


Mathematics, 21.06.2019 22:30, jadeochoa4466
Aflagpole broke in a storm. it was originally 8 1 81 feet tall. 2 8 28 feet are still sticking straight out of the ground, where it snapped, but the remaining piece has hinged over and touches the ground some distance away. how far away is the end of the pole from the base of the pole along the ground?
Answers: 1

Mathematics, 22.06.2019 00:30, ashiteru123
What is the value of x? enter your answer in the box. x =
Answers: 1

Mathematics, 22.06.2019 00:30, hannahpalacios101
36x2 + 49y2 = 1,764 the foci are located at: a) (-√13, 0) and (√13,0) b) (0, -√13) and (0,√13) c) (-1, 0) and (1, 0)
Answers: 1
Do you know the correct answer?
Un elemento tiene las siguientes abundancias naturales y masas isotópicas: 90,92% de abundancia con...
Questions in other subjects: