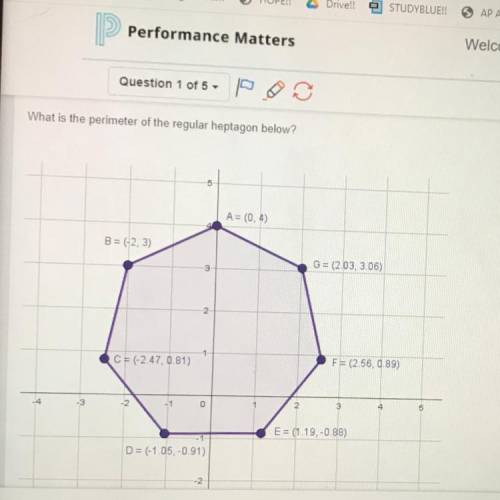
Which of the following statements are true? (select all that apply.) a quasi-static process is one in which the system is never far from being in equilibrium. when a system can go from state 1 to state 2 by several different processes, the amount of heat absorbed by the system will be the same for all processes. the internal energy of a given amount of an ideal gas depends only on its absolute temperature. when a system can go from state 1 to state 2 by several different processes, the work done on the system will be the same for all processes. when a system can go from state 1 to state 2 by several different processes, the change in the internal energy of the system will be the same for all processes. for any substance that expands when heated, its cp is greater than its cv.
Answers: 2