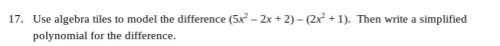Concentration of NO2 = 11.95

Mathematics, 08.01.2021 01:00, krystalhurst97
For the current reaction, 2NO2 ↔ N2O4, we have:
Kc = ![\frac{[N_{2} O_{4}]}{[NO_{2}]^{2} }](/tpl/images/2047/8468/2ef86.png)
Concentration of NO2 = 11.95
Concebtration of N2O4 = 6.05
Based on the current concentrations of NO2 and N2O4, what is Kc?

Answers: 3
Other questions on the subject: Mathematics

Mathematics, 21.06.2019 16:30, hasshh
Identify the converse of the following conditional: if a point is in the first quadrant, then its coordinates are positive. if the coordinates of a point are not positive, then the point is not in the first quadrant. if the coordinates of a point are positive, then the point is in the first quadrant. if a point is in the first quadrant, then its coordinates are positive. if a point is not in the first quadrant, then the coordinates of the point are not positive.
Answers: 2

Mathematics, 21.06.2019 19:30, noahdeem135
Asurvey of 2,000 doctors showed that an average of 3 out of 5 doctors use brand x aspirin. how many doctors use brand x aspirin
Answers: 1

Mathematics, 21.06.2019 23:20, tsedeneyaalemu2924
Write the equations in logarithmic form 10^3=1,000
Answers: 1

Mathematics, 22.06.2019 01:20, stastnylindsey
Write 5 in the form of a/b using integers to show it as a rational number
Answers: 1
Do you know the correct answer?
For the current reaction, 2NO2 ↔ N2O4, we have:
Kc =
Concentration of NO2 = 11.95
Concentration of NO2 = 11.95
Questions in other subjects:


Mathematics, 03.08.2019 12:00





Spanish, 03.08.2019 12:00


Physics, 03.08.2019 12:00


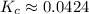
![\displaystyle K_c = \frac{[N_2O_4]}{[NO_2]^2}](/tpl/images/1020/5386/82efd.png)
![\displaystyle K_c = \frac{[6.05]}{[11.95]^2}](/tpl/images/1020/5386/714da.png) Exponents:
Exponents: ![\displaystyle K_c = \frac{[6.05]}{[142.803]}](/tpl/images/1020/5386/9a115.png) Divide:
Divide: 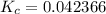 Round (Sig Figs):
Round (Sig Figs):