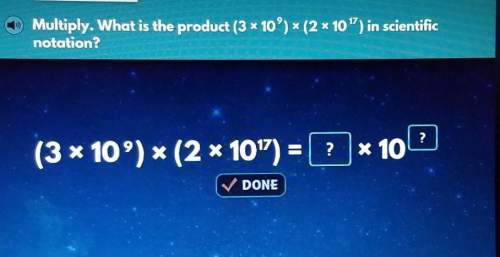
Mathematics, 26.11.2020 14:00, claytonhopkins
A sample of metal that has a mass of 12.48 g is heated to 99.0∘C and then dropped into 50.0mL of 25∘C water, causing the temperature of the water to become 28.1∘C. What is the specific heat of the metal? Round your answer to two decimal places. Use 4.184Jg∘C for the specific heat of water. Convert mL of water to grams given the Density of water = 1 g/mL.

Answers: 1
Other questions on the subject: Mathematics

Mathematics, 21.06.2019 22:20, jonestmoney381
Jimmy can run 3.5 miles in 20 minutes. how far can ne run in one hour and ten minutes?
Answers: 1


Mathematics, 22.06.2019 01:50, iiMxlissaii
Grandpa ernie is shrinking! over the past 4 years his height decreased by a total of 2.4 cm. it decreased by the same amount each year. what was the change in grandpa ernie's height each year
Answers: 2

Mathematics, 22.06.2019 02:20, IkweWolf1824
Find the volume of the wedge cut from the first octant by the cylinder z=12-3y^2 and the plane x+y=2.
Answers: 1
Do you know the correct answer?
A sample of metal that has a mass of 12.48 g is heated to 99.0∘C and then dropped into 50.0mL of 25∘...
Questions in other subjects:




Social Studies, 05.05.2020 22:03

Biology, 05.05.2020 22:03

Mathematics, 05.05.2020 22:03



Advanced Placement (AP), 05.05.2020 22:03

Mathematics, 05.05.2020 22:03