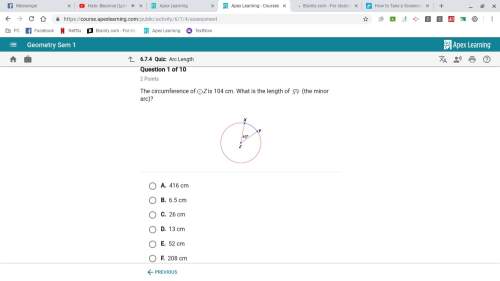
Mathematics, 04.11.2020 09:00, dkline89
Rust results from iron’s reaction to oxygen. An iron nail gains mass when it rusts. How does this reaction support the law of conservation of mass?
The mass of the rusted nail equals the mass of iron and the oxygen from the air it reacted with to form the rust.
The mass of the rusted nail increases because iron attracts more protons from the air.
The increased mass of the rusted nail is an exception to the law of conservation of mass since the rusted nail’s mass increases.
The increased mass of the rusted nail results from the rearrangement of protons and neutrons within oxygen, according to the law of conservation of mass.

Answers: 2
Other questions on the subject: Mathematics




Mathematics, 21.06.2019 23:30, shady1095
Asap (i need to finish this quick) graph complete the sequence of transformations that produces △x'y'z' from △xyz. a clockwise rotation ° about the origin followed by a translation units to the right and 6 units down produces δx'y'z' from δxyz.
Answers: 1
Do you know the correct answer?
Rust results from iron’s reaction to oxygen. An iron nail gains mass when it rusts. How does this re...
Questions in other subjects:




Mathematics, 15.12.2020 22:10

Mathematics, 15.12.2020 22:10

English, 15.12.2020 22:10