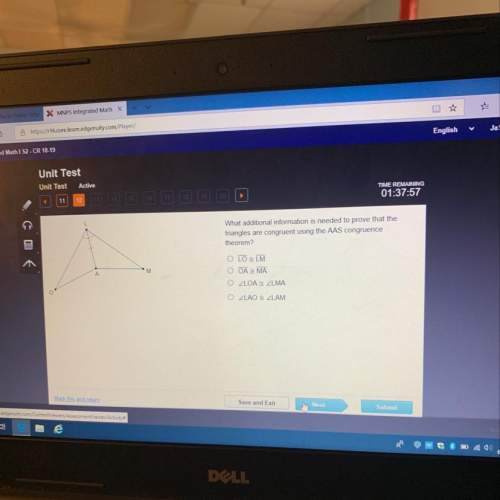
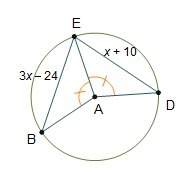
Mathematics, 27.06.2020 03:01, saeedalr366
Please help 1. rubidium has two isotopes, 72.2% rubidium-85, and 27.8% rubidium-87. Calculate the average atomic mass of rubidium. 2. Bromine also has two isotopes: 50.7% is bromine-79 and 49.3% is bromine-81. Can you estimate the average atomic mass of bromine without using a calculator? 3. What is the average atomic mass of fluorine, whose only naturally occurring isotopes has an atomic mass of 19?

Answers: 1
Other questions on the subject: Mathematics

Mathematics, 21.06.2019 16:40, Alex9089435028
The sum of two numbers is 86, and their difference is 20. find the two numbers
Answers: 2



Mathematics, 21.06.2019 22:00, dorothybean
Describe how you can act financially responsible.
Answers: 1
Do you know the correct answer?
Please help 1. rubidium has two isotopes, 72.2% rubidium-85, and 27.8% rubidium-87. Calculate the av...
Questions in other subjects:

Mathematics, 29.10.2020 17:20




History, 29.10.2020 17:20





Biology, 29.10.2020 17:20